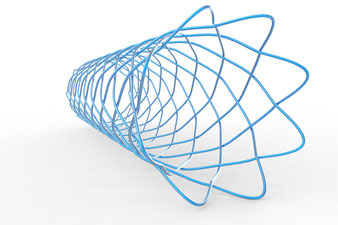
Stent design and FEA
Stent design (laser cut)
our strengths
- Stent conception
- Stent design (2D and 3D)
- Provide production drawings (laser cut DXF drawing)

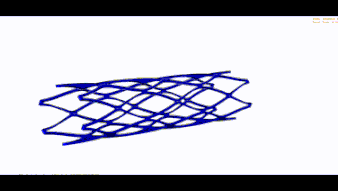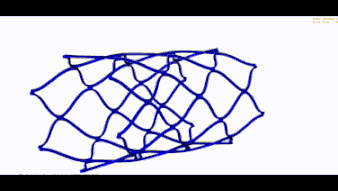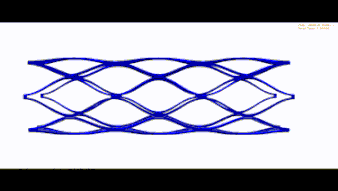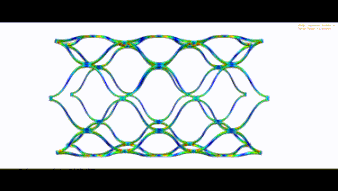
Stent optimization and validation
The following parameters can be optimized:
- Radial force
- Flexibility in the vessel
- Distal and Proximal Stent side
- Crimp behavior
- Expansion behavior
- Fatigue
The following international standards are used in this context:
ISO 25539-1: Cardiovascular implants – Endovascular devices – Part 1: Endovascular prostheses
ISO 25539-2: Cardiovascular implants – Endovascular devices – Part 2: Vascular stents
ISO 25539-3: Cardiovascular implants – Endovascular devices – Part 3: Vena cava filters
ASTM F2514: Finite Element Analysis (FEA) of Metallic Vascular Stents Subjected to Uniform Radial Loading
ASTM F2477: Standard Test Methods for in vitro Pulsatile Durability Testing of Vascular Stents
ASTM F2942: Standard Guide for in vitro Axial, Bending, and Torsional Durability Testing of Vascular Stents
ASTM F3067: Guide for Radial Loading of Balloon Expandable and Self Expanding Vascular Stents
ASTM F2079: Elastic Recoil of Balloon-Expandable Stents
ASTM F3211: Fatigue-to-Fracture (FtF) Methodology for Cardiovascular Medical Devices.
FDA Guidance for Industry and FDA Staff: Non-Clinical Engineering Tests and Recommended Labeling for Intravascular Stents and Associated Delivery Systems. April 18, 2010
FDA Guidance for Industry and FDA Staff: Reporting of Computational Modeling Studies in Medical Device Submissions. September 21, 2016
Heart Valves:
ISO 5840-3: Cardiovascular implants – Cardiac valve prostheses – Part 3: Heart valve substitutes implanted by transcatheter techniques
ISO 5910: Cardiovascular implants and extracorporeal systems – Cardiac valve repair devices



Stent FEA (Braided and Lasercut)
We take the numerical proof of your stent and carry out the following simulations:
- Radial force (in the intended used area of the stent)
- Crimping (radially compressing up to catheter ID)
-
- Use heat treated material parameters (eg: NiTinol) !!!
- Expand (to the desired diameter)
- Stent fatigue (stent fatigue strength in the human vessel)
- Stent deployment (release)
- Stent foreshortening (axially compressing of the stent)
The following international standards and regulations are used in this context:
ISO 25539-1: Cardiovascular implants – Endovascular devices – Part 1: Endovascular prostheses
ISO 25539-2: Cardiovascular implants – Endovascular devices – Part 2: Vascular stents
ISO 25539-3: Cardiovascular implants – Endovascular devices – Part 3: Vena cava filters
ASTM F2514: Finite Element Analysis (FEA) of Metallic Vascular Stents Subjected to Uniform Radial Loading
ASTM F2477: Standard Test Methods for in vitro Pulsatile Durability Testing of Vascular Stents
ASTM F2942: Standard Guide for in vitro Axial, Bending, and Torsional Durability Testing of Vascular Stents
ASTM F3067: Guide for Radial Loading of Balloon Expandable and Self Expanding Vascular Stents
ASTM F2079: Elastic Recoil of Balloon-Expandable Stents
ASTM F3211: Fatigue-to-Fracture (FtF) Methodology for Cardiovascular Medical Devices.
FDA Guidance for Industry and FDA Staff: Non-Clinical Engineering Tests and Recommended Labeling for Intravascular Stents and Associated Delivery Systems. April 18, 2010
FDA Guidance for Industry and FDA Staff: Reporting of Computational Modeling Studies in Medical Device Submissions. September 21, 2016
Heart Valves:
ISO 5840-3: Cardiovascular implants – Cardiac valve prostheses – Part 3: Heart valve substitutes implanted by transcatheter techniques
ISO 5910: Cardiovascular implants and extracorporeal systems – Cardiac valve repair devices